(i) Project Title: Knee Joint Simulation
Project Brief
- A detailed FE model of the human knee joint that includes fiber-reinforced cartilage, bone, and ligaments was developed.
- Physiological loading conditions were applied.
- This model will be used to study fracture mechanics of cartilage and bone under different loading conditions.
- The next step involves, a multiscale simulation of the knee cartilage biomechanical response.
Skills Needed
Material Modeling
FE Simulation
Programming
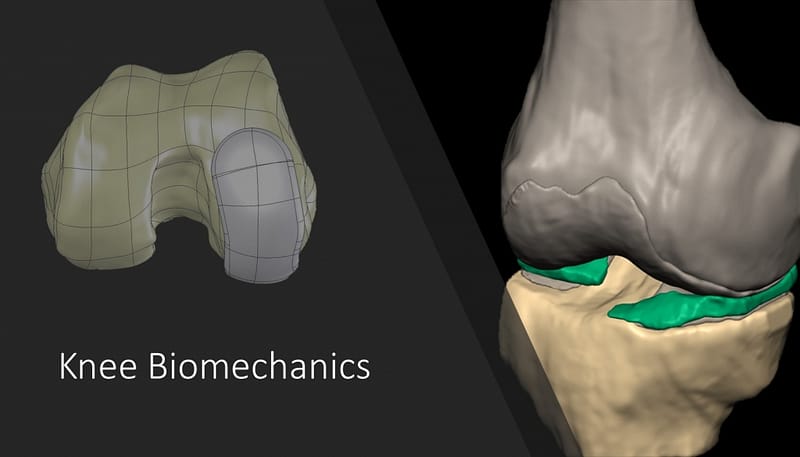
Model Description
Detailed geometry of the human knee joint was reconstructed from MRI images. After segmentation, the geometry was cleaned from spikes, holes and sharp edges. The reconstructed geometry then meshed: femur and tibia comprised of cancellous and cortical bones. Due to the complexity of geometry and to keep it without any correction or simplification, the cortical bone was meshed using 3-node triangular shell elements of 1 mm thickness. Four-node tetrahedral solid elements were used to mesh the cancellous. 8-node brick elements were used to mesh cartilage and meniscus. A 3 mm thickness was assigned to both femur and tibia cartilages. A capsule was created to connect two adjacent contact surfaces. Collagen fibers were corporate in the model using a UMAT subroutine with controlled spatial orientation. The cartilage was modelled as a biphasic material, consisting of 80% solid matrix and 20% interstitial fluid.



Team Leaders
(ii) Project Title: Automated FEM Model Development
The field of engineering was revolutionized by adapting numerical methods to solve math and physics equations. Soon, classical analytical methods, such as Fourier Transform, for solving partial differential equations were either replaced or integrated into more advanced numerical methods such as finite element analysis (FEA) to solve even more complicated problems. The application of FEA was increased significantly with the introduction of computers by which abundant calculations were performed with minimum human error and maximum reliability. For years FEA, in solids, and CFD, in fluids, analyses have been the focus of researchers. With that, more complex problems at larger scales were submitted for analysis which, depending on the complexity of the model and application, may take anywhere from minutes to days. The complexity and time-consuming nature of the current workflow for some applications, such as developing patient-specific FE models, makes it impractical to apply conventional FE for computational analysis, specifically in time-sensitive applications. We hypothesize that a paradigm-changing machine learning (ML) solution has the potential to revolutionize the FEA to render the stress analysis in a few seconds for time-sensitive clinical applications, which require fast diagnosis and treatment planning.
The Solution
Automatic Model Development
Geometry is the pillar of every FE model. The “shape” and “appearance” are two key playing factors in object segmentation. In MRI or CT imaging of knee joints, the low-level appearance information might be weak or misleading. Thanks to the strong shape characteristics of the knee joint, the shape priors play a more important role to guide a correct segmentation. To effectively reconstruct an accurate shape model, the following challenges should be addressed: 1- complex shape variations; (2) error associated with the image appearance cues; and (3) preserving the local details of the input shape. Non-parametric methods could be a solution to tackle these three challenges in a unified framework (Zhang et al., 2011a, 2012). The non-parametric methods benefit from considering a general distribution model, modeling the gross error, and exploiting information from all training shapes.
AI-FEA
Since the biological tissue shape does not variate significantly from patient to patient, there is a potential for training a machine-learning algorithm to map the shape code to stress code. The neural network was shown as an effective method of approach for this purpose.
Zhang, W., et al. 2011b. Estimating patient-specific shape prior for medical image segmentation. In: International Symposium on Biomedical Imaging. IEEE, 1451–1454.
Zhang, S., et al. 2012. Towards robust and effective shape modeling: sparse shape composition. Medical Image Analysis 16, 265–277.
We are looking for an automated method to develop patient-specific FE knee model and predict the state of stresses and strains in the knee components as a fast and accurate surrogate of FEA. Currently, image segmentation, geometry and mesh development, and conducting FEA of full knee model may take few days. Trained deep learning models could generate the cartilage stress distributions on a scale of few seconds, consistent with and magnitudes faster than FEA. Such a method has significant application in clinics where predicting the mechanobiological response of human organs in a timely manner is important.
Intervertebral Disk Simulation
Fiber Reinforced Biphasic IVD
– Including the effect of IVD osmolarity
– Disk fibers were modelled implicitly using a Python code.
– Structured mesh
– Fluid-solid interactio

FE Model Characteristics
A 3D FE model of the C2-C3 FSU was created. Bony components geometry was reconstructed from 1 mm interval CT-Scans. Each vertebra comprises cancellous and cortical bones and articular facets. The complex geometry of cortical bone was meshed using 3-node triangular shell elements. Four-node tetrahedral solid elements were used to mesh the cancellous bone. Facet cartilages and endplates were meshed with 8-node brick elements. Two capsules were created to connect the adjacent facets on both sides

Results
The mechanical response of the C2-C3 spine was studied under a compression ramp, cyclic compression, and bending moments. The effect of IVD material model on the spine load-sharing was studied was investigated by assigning linear elastic, hyperelastic, and biphasic material properties to the AF, NP, and endplates.